Ten Steps Program to Boost Brain Health Step 5:
Nurturing the Gut-Brain Axis: A Voyage Towards Ageless Brain Health
Introduction:
Welcome back. On our last foray into preserving brain function and restring cognitive function, dated October 31, 2023, (1) ‘The Crucial Role of Vitamin D in Brain Health,’ continued our discussion on maintaining and preserving the
10 Steps to a Healthy Brain: (2)
- Keep Your Blood Sugar Balanced
- Eat Healthy Fats
- Get Adequate and Restful Sleep
- Enough (but not too much) Vitamin D3 is Essential for the Brain to Function
- Get Your Gut in
- Maintain Adequate Methylation
- Balance Your Hormones
- 6 Fixes for A Healthy Heart
- Exercise
- Lifetime Learning
Preamble
How do you resolve these scenarios between want and need?
You are in a delicatessen. You have narrowed your choice to a corned beef sandwich on rye with cole slaw and Russian dressing versus a salad with some grilled chicken and a vinaigrette dressing. You chose the sandwich. It was heaven until an hour later when your stomach rumbled. Guilt, much?
That afternoon, you come across an opportunity to “invest” in Vandalay Industries out of Wilkes Barre, Pa. (Not a real company.) They’ve introduced a “surefire” winner, a robot butcher. Vandalay is an investment trust. For every $1000 invested, you are promised
$300 a week in dividends for the rest of your life. Who wouldn’t jump at this massive “guaranteed” return for life? But a nagging rumble in your stomach says, “Hold on, sailor. If it’s too good to be true…”
Lastly, over the past week, you’ve felt impending doom. You are anxious, you notice more hair in the shower drain, your skin has scaly patches, your fingernails are cracking and breaking, you are exhausted all day long, it is 80 degrees outside, and you are wearing a sweater, and even your eyebrows are thinning at the edges.
“What’s going on, Doc?” you ask your family GP, Dr. Melnitz. He hems and haws, orders blood tests, and tells you it is in your head; nothing’s wrong.
“Stress,” Melnitz says. “If it doesn’t clear up, go see a specialist.”
That nagging in your gut again tells you you are being gaslighted. After a year of visiting medical specialists, reiki therapists, psychiatrists, psychologists, and an ashram in India, you feel worse, not better. Something is wrong.
“You’re my last hope, Dr. C.”
“My shoulders aren’t that wide,” Dr C replies.
Dr. C does his hormone optimization thing, and lo and behold, your thyroid antibodies, indicative of an autoimmune thyroid disorder, are off the charts.
“It usually takes five years before symptoms become noticeable, then another five years before blood tests become severe enough to render a diagnosis. By the way, the first symptom is usually an unexplained anxiety.”
Deep down, you knew something was wrong. You know the salad was better for your long-term health than the fatty sandwich. You knew the “investment” was a scam. Your instincts didn’t let you accept that “the blood work is normal; it was all in your head.”
In the old days, before cell phones, the internet, and instant messaging, we called it ‘gut instinct.’ Thomas Edison’s gut instinct, for instance, despite thousands of failed attempts, ultimately led to the successful invention of the incandescent light bulb. (3)
Henry Ford’s gut instinct created an affordable automobile accessible to all Americans. Ford followed his intuition, despite the naysayers, as he developed the assembly line method of mass production and, ultimately, the Model T. (4)
The Microbiome and Brain Health Connection
It turns out that ‘gut instinct,’ the intuitive sense that often guides us in decision-making, is more than just a metaphor. We have a ‘second brain,’ termed the Enteric Nervous System (ENS). The enteric nervous system is a network of at least 600 million nerve cells (neurons) in the walls of the intestines, regulated chemical messengers, known as neurotransmitters, as similarly produced in the brain. (5)
Wrapped in and around the gastrointestinal system, the enteric nervous system is a living, breathing, complex biosphere that substantially impacts our overall health. The functions of the ENS range from the propulsion of food to nutrient handling, blood flow regulation, and immunological defense. (6)
The prime function of the GI tract, of course, is managing digestion. However, the presence of this brain-like complexity is no coincidence. Our ENS constantly communicates with the brain properly via the vagus nerve. Chemical reactions flow back and forth between the gut and the brain, influencing decision-making, mood, and general well-being. (7)
When depleted, serotonin is the neurotransmitter widely regarded as a root cause of major and minor depression. The enteric nervous system produces 95 percent of the body’s serotonin (8). Thus, as the ENS goes, so does our physical and mental health.
A Healthy Microbiome is an Anti-Aging Device.
But wait, there is more! The enteric nervous system is controlled by a vast array of bacteria, viruses, and protozoans found mainly in the gut, termed the microbiome. The human gastrointestinal (GI) tract contains an abundant and diverse microbial community of more than 100 trillion microorganisms per person. (9)
The density of bacterial cells in the colon is estimated to be 1011 to 1012 per milliliter, making the colon one of the most densely populated microbial habitats. (10) The microbiome encodes over 3 million genes, whereas the human genome contains approximately 23,000 genes. (11)
A healthy microbiome contributes to the body’s homeostasis by aiding digestion, nutrient absorption, and short-chain fatty acid (SCFA) production. (9) An imbalance in microbial composition, alteration in microbial metabolic activity, or disruption in bacterial distribution within the gut leads to “dis-ease,” defined as dysbiosis.
The Microbiome in Action
1. Inflammation
Microbial gut flora produces anti-inflammatory compounds and promotes the production of anti-inflammatory regulatory T-cells. Examples include:
- Bifidobacterium has short-chain fatty acids like acetate that nourish intestinal cells. Bifidobacterium induces Treg cells and suppresses TNF-alpha and IL-8 production. (10)
- Lactobacillus species secrete lactic acid and other metabolites that inhibit inflammatory cytokines. rhamnosus and L. casei induce
anti-inflammatory Treg cells and IL-10 production. (11)
- Faecalibacterium prausnitzii produces butyrate. Butyrate nourishes colonocytes and suppresses NF-κB activation and IL-6 Linked to low levels of butyrate are inflammatory bowel diseases. (12)
- Akkermansia muciniphila maintains intestinal barrier integrity through mucus production and limits immune cell recruitment and IL-6
(13)
- Clostridium clusters IV, XIVa, and XVIII include major butyrate producers that nourish colonocytes and suppress inflammation. (14)
- Bacteroides fragilis produces an immune system polysaccharide, which directs and induces anti-inflammatory IL-10-producing Treg cells. (15)
- Helicobacter pylori generates high levels of vacuolating cytotoxin A, dampening immune responses and protecting the host against allergies and asthma. (16)
These common bacteria achieve their anti-inflammatory effects through metabolite production, modulation of barrier function, and immune cell regulation.
The remedy for dysbiosis is administering probiotics and prebiotics to rebalance the microbiome. Probiotics reintroduce beneficial bacteria to the GI system. Prebiotics provide the “food” for healthy microbes already present. (17)
2. The Gut-Brain Axis
Incredibly, the ‘gut feeling’ we experience in times of danger or stress is a living, breathing entity. The enteric nervous system controls the GI system, which is governed by the microbiome.
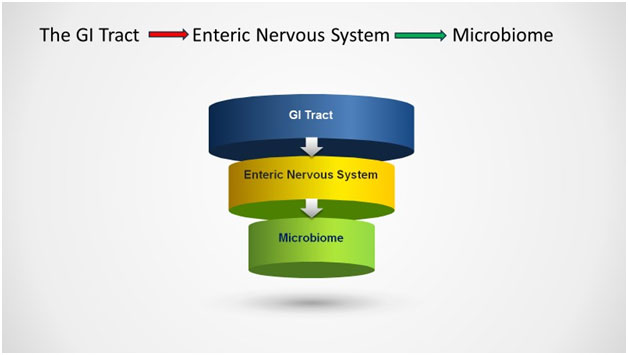
Our gut-microbiome-brain axis produces GABA, serotonin, dopamine, acetylcholine, and short-chain fatty acids. These neurotransmitters enter our systemic circulation and cross the blood-brain barrier to directly influence brain function. (18)
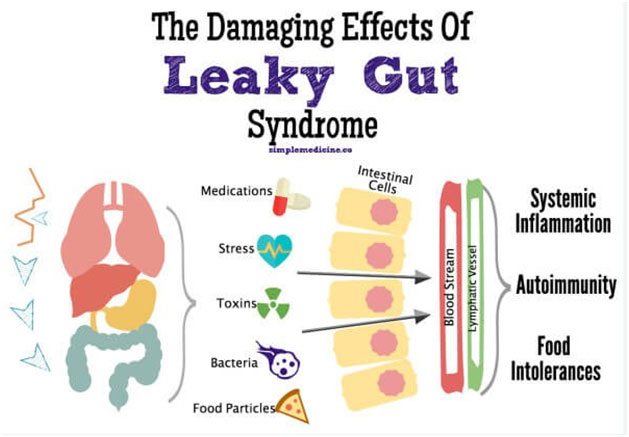
Zonulin, a sticky, glue-like substance, holds the lining cells of the GI tract in place. It protects the GI tract and, by proxy, cerebral function from intestinal hyperpermeability (the so‐called “leaky gut”) and chronic disease.
80% of our immune system resides in the GI mucosal lining. Damage to this barrier disrupts the microbiome, leading to immune dysfunction. (18) Damage to the immune system affects the whole body, particularly the brain. (19)
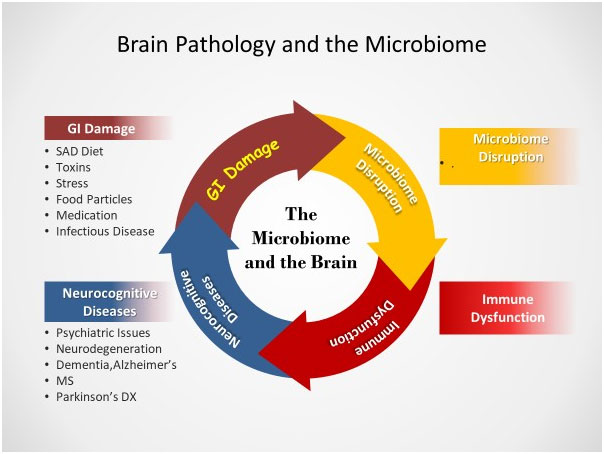
The etiology of a disrupted microbiome primarily begins with the inflammatory, ‘Standard American (SAD) Diet. ‘ Throw in 80-100,000 new environmental toxins introduced in the past fifty years, the need to ‘go-go-go’- i.e., sleep deprivation, alcohol, and drug abuse, and family and financial stressors- and we have the perfect storm of chronic disease.
In the genre of dietary indiscretions, gluten, dairy, corn, soy, eggs for sensitive people, and sugar are the bad guys.
Gluten, a protein found in modern-day barley, rye, and triticale, a cross between wheat and rye, is a significant stressor of the small intestine’s junctional walls. As previously noted, weakened intestinal walls create openings to allow toxins, microbes, and food particles a clear path to the bloodstream.
(60)
Our immune system misinterprets the out-of-place nutritional and biochemical data received in the bloodstream. This toxic stew infiltrates our blood and lymphatic streams and rejects our tissue. Antibodies form to protect ourselves from our tissues, perceived as foreign invaders. This antibody reaction to ourselves, termed ‘autoimmune,’ is our (misguided) attempt to fight against the perceived ‘invaders.’ (20)
The microbiome is a critical barrier keeping these autoimmune reactions in check. The microbiota augments intestinal innate immunity by secreting antimicrobial peptides, proteins (AMPs), and mucus and providing pathogen colonization resistance. The microbiota also alters the body’s response to pathogens and can either lessen or enhance the efficiency of drugs and the immune system. (21)
When disrupted, the microbiome reacts by releasing proinflammatory proteins (named cytokines) with local and systemic effects on the immune system. These inflammatory chemicals create breaches in mucosal barriers while generating commensal‐specific memory T cells and autoantibodies. (22)
The gut microbiota delicately balances homeostasis to avert Hashimoto’s thyroiditis, rheumatoid arthritis, and ninety other recognized autoimmune diseases. (23)
The vagus nerve is our direct communication pathway between gut microbes and the brain. Stimulation of the vagus nerve activity influences the activity of the neurotransmitters norepinephrine and serotonin implicated in mood disorders. (24)
Gut microbes are crucial in regulating tryptophan metabolism and influencing the amount transported to the brain. Once inside the brain, tryptophan converts into serotonin, a neurotransmitter infamously known to be deficient in conditions of anxiety and depression. (25)
The microbiome interacts with the immune system, impacting neuroinflammation. (26)
Alzheimer’s disease, Parkinson’s, and multiple sclerosis are results of microbiome-induced neurodegeneration. (27)
Specific microbes involved in microglial activity include:
- Bacteroides fragilis (28)
- Lactobacillus rhamnosus (JB-1) (29)
- Clostridium butyricum (30)
- Bifidobacterium longum (31)
On the positive side, gut bacteria produce neurotrophic factors like BDNF and NGF that support neuron health and function. Declining levels contribute to neurodegeneration.
(32)
The entire mechanism is akin to a complex puzzle, much like a “who shot John” situation as depicted by Judge Judy. The gut-brain axis represents a sophisticated,
two-way communication system linking the gastrointestinal tract with the central nervous system. Gut microbiota plays a pivotal role in managing this axis, profoundly impacting neurotransmitter production, immune system responses, intestinal barrier health, and neurotrophic factors. Each of these components significantly influences brain functionality and the likelihood of developing neurodegenerative diseases.
Added Bonus-Other Benefits of an Intact, Functioning Microbiome
- Metabolic Efficiency
A well-balanced microbiome efficiently processes fiber into short-chain fatty acids (SCFAs). These SCFAs are crucial in regulating energy metabolism and enhancing insulin sensitivity. (33)
2. Oxidative Stress
Gut bacteria produce or induce antioxidant compounds to reduce aging and degenerative disease stressors. (34)
Lactobacillus species act against oxidative stress-related tumor development. (35) Lactic acid bacteria (LAB) contain antioxidant properties to improve insulin resistance and treat diabetes, Alzheimer’s disease, Parkinson’s disease, obesity, liver diseases, and tumors and restore the balance of good and bad bacteria in your gut. (36-37)
3. Telomere Length
Telomeres, structures made from DNA and proteins found at the ends of chromosomes, are required for cell division. (38) Telomeres play a vital role in preserving the information in our genome by protecting chromosomes from degradation and aberrant regeneration, i.e., “aging.” Telomere length is longest at birth, shortens rapidly until adolescence, and then less rapidly until old age. Longer telomeres are generally associated with longer lifespans. (39)
A healthy microbiome protects telomeres and genomic integrity from cellular stress. (40)
Shorter telomere length results in reconfiguration of the gut microbiome. Gut telomere shortening exacerbates age-dependent effects over the entire organism through its microbial components. (41)
As you probably deduced, “good” microbiota that preserves or lengthens telomeres results from consuming legumes, nuts, seaweed, fruits, 100% fruit juice, dairy products, and coffee. Consumption of alcohol, red meat, or processed meat damages the microbiome, resulting in telomere shortening. (42)
4. Immune System Modulation
A healthy, diverse microbiome helps slow or prevent immune dysfunction of aging.
Age-associated shifts in the gut microbiome contribute to an increased predisposition of aged individuals to cardiovascular diseases, cancer, obesity, diabetes, and neurodegenerative diseases. (43)
5. Detoxification
The liver works with the gut microbiota to detoxify harmful substances. An imbalance in the microbiome impairs this process, leading to the accumulation of damaging substances. (44)
Working with the gut microbiota, the liver plays a crucial role in detoxifying harmful substances. Any imbalance in the microbiome, labeled dysbiosis, leads to the accumulation of: (45)
- Lipopolysaccharides (LPS) are bacteria-produced endotoxins that lead to inflammation and diseases like sepsis if they enter the bloodstream. (46)
- Ammonia: Produced by the bacterial breakdown of proteins, excess ammonia levels lead to brain swelling (cerebral edema), pressure around the brain (intracranial hypertension), brain herniation (when brain tissue moves), coma, and death. (47)
- Trimethylamine N-oxide (TMAO) is created by gut bacteria when one eats animal products like red meat, eggs, fish, and Elevated levels of TMAO result in inflammatory conditions, including heart disease, type 2 diabetes, chronic kidney disease, cancer, and liver disease. (48)
- Short-chain fatty acids (SCFAs): While beneficial in moderation, overproduction of SCFAs leads to an acidic environment indicating bacterial overgrowth. SCFA excess leads to gut microbiome dysbiosis, obesity, hypertension, cardiometabolic disease risk factors, increased gut permeability, metabolic dysregulation, inflammation, altered levels of neurotransmitters, and intracellular potassium levels. (49)
- Secondary bile acids: Gut bacteria convert primary bile acids into secondary ones. Secondary bile acids contribute to colorectal cancer and gallstone formation. (50)
6. Hormonal Regulation
- Estrogen: A collection of bacteria in the gut, termed the “estrobolome,” metabolizes and modulates estrogen levels in the The estrobolome plays
a vital role in breaking down estrogen into a form that can be eliminated from the body. Disruptions in the estrobolome lead to estrogen excess syndromes manifesting as weight, libido, blood sugar, fibrocystic breast disease, ovarian cysts, endometriosis, uterine fibroids, and gynecological cancers. (51)
- Cortisol: The microbiome interacts with the hypothalamic-pituitary axis (HPA), affecting the body’s response to stress by influencing the production of the stress hormone cortisol. Stress effects on the GI system include changes in intestinal motility, gut barrier permeability, and enhanced gut sensitivity. (52)
Note that the signals between the central nervous system and the gut microbiome are bi-directional. Just as the microbiome can affect cortisol levels, elevated cortisol levels due to outside pressures influence the function and makeup of the microbiome. (53)
- Insulin: Gut microbiota affects host metabolism and obesity via gut barrier integrity, production of metabolites affecting satiety and insulin resistance, epigenetic factors, metabolism of bile acids, and subsequent changes in metabolic signaling. (54)
Higher levels of the bacterium Coprococcus tend to have higher insulin sensitivity. Higher levels of the bacterium Flavonifractor tended to have lower insulin sensitivity. (55)
- Appetite: Alterations in the gut microbiota influence appetite by affecting the secretion of appetite-related hormones leptin and ghrelin. (56)
- Serotonin, GABA, serotonin, norepinephrine, dopamine, acetylcholine, and melatonin are directly synthesized by the gut microbiota. Dopamine and norepinephrine are involved in gut-brain communication. (57)
- Caloric Restriction Mimicry: Specific gut bacteria can replicate the benefits of caloric restriction, a recognized method for prolonging This calorie-restricted behavior is achieved by impacting energy metabolism and interacting with pathways linked to increased longevity. (58)
How to Repair an Injured Microbiome:
- Use Antibiotics with Caution and Only When Necessary: Antibiotics are essential for combating bacterial infections but may lack selectivity in the bacteria they eliminate. Antibiotics also destroy beneficial gut bacteria, potentially causing dysbiosis. It is important to use antibiotics judiciously and only when necessary. (59)
- Probiotics and Prebiotics: Found in fermented foods like yogurt, sauerkraut, and kimchi or taken as a supplement, probiotics are beneficial bacteria that help restore balance to your gut Prebiotics, derived from dietary fiber the body can’t digest, including bananas, onions, garlic, leeks, asparagus, artichokes, and whole grains, are food for these bacteria. (60-61)
- Fecal Transplantation: Used to restore the balance of bacteria in a patient’s gut, Fecal transplants are used to treat difficult-to-treat diseases like recurrent C. difficile infection. The procedure involves the transfer of fecal bacteria from a healthy individual to an ill one. (62)
- Dietary Changes: The gut microbiome responds to what you A diet high in fiber, fruits, vegetables, lean proteins, and healthy fats encourages a diverse microbiome. Conversely, a diet high in processed foods, sugars, and unhealthy fats leads to a less diverse microbiome. (63)
- Exercise: Regular physical activity enhances the number of beneficial microbial species, enriches the microflora diversity, and improves the development of commensal bacteria. (64)
- Bone Broth: Bone broth is rich in collagen and other nutrients that help repair the gut lining. (65)
- Time: Healing the gut microbiome doesn’t happen Depending on the damage, it can take months for the gut to recover fully. During this time, it’s essential to feed your body with gut-friendly foods, get plenty of rest, manage stress, and avoid any triggers that could cause further damage. (66)
Summary
Ten Steps Program to Boost Brain Health,” Step 5:
- **Gut-Brain Axis Importance**: We delve into the pivotal importance of the
gut-brain axis in maintaining overall health, emphasizing the Enteric Nervous System
(ENS) as an integral ‘second brain’ nestled within the intestines. This remarkable system significantly influences our decision-making processes, mood regulation, and general well-being, underscoring its essential contribution to our holistic health.
- **Microbiome’s Role in Brain Health**: The human microbiome, consisting of trillions of microorganisms, is crucial for maintaining brain health. It influences neurotransmitter production, immune responses, and intestinal barrier health.
- **Serotonin Production**: 95% of the body’s serotonin, a key neurotransmitter affecting mood, is produced in the gut, linking gut health directly to mental health.
- **Inflammation and Immune Function**: The microbiome plays a vital role in producing anti-inflammatory compounds and regulating immune function, crucial for preventing chronic diseases.
- **Dietary Impact on Microbiome**: The Standard American Diet (SAD) and environmental toxins contribute to microbiome disruption, leading to deleterious health
- **Autoimmune Reactions and Gut Health**: A disrupted microbiome leads to autoimmune reactions, where the body mistakenly attacks its own tissues, highlighting the importance of a balanced microbiome.
- **Additional Benefits of a Healthy Microbiome**: The article lists several benefits of a healthy microbiome, including metabolic efficiency, oxidative stress reduction, telomere length maintenance, immune system modulation, and detoxification.
- **Hormonal Regulation**: The microbiome regulates key hormones estrogen, cortisol, and insulin, affecting various aspects of health.
- **Caloric Restriction Mimicry**: Certain gut bacteria replicate the benefits of caloric restriction. Known for prolonging lifespan, intermittent fasting impacts energy metabolism and longevity pathways.
- **Repairing the Microbiome**: We discuss strategies for repairing an injured microbiome, including cautious antibiotic use, incorporating probiotics and prebiotics, fecal transplantation, dietary changes, exercise, and consuming bone broth.
Conclusion
A healthy gut is essential for a healthy brain. The microbiome plays an enormous role in keeping us functioning at our best. It takes consistent effort and, at times, lifestyle
changes to keep the microbiome functioning optimally. A healthy microbiome is crucial for a healthy life.
Eating fiber-rich foods, limiting processed foods, introducing fermented foods, limiting stress as much as possible, getting enough sleep, exercising regularly, and participating in mindfulness activities like yoga are essential in repairing a damaged microbiome.
Building a solid foundation of health starts with taking control of your gut. Ultimately, a healthy microbiome leads to a better mood.
Today, right now, take that first step now to make your brain healthier and happier!
xxxxx
References
- W., ‘The Crucial Role of Vitamin D in Brain Health.’ https://longevinex.com/the-role-of-vitamin-d-in-brain-health/ October 31, 2023
- https://docs.google.com/document/d/14Ym_CTkktfYQk1WjeCVg6bVbKqQP3DA 6cAVMaL97fqc/edit
- https://www.quora.com/What-are-some-examples-of-instinctive-decisions-that-ha ve-proved-decisive-for-better-or-for-worse-in-history)
- https://killerinnovations.com/is-intuition-required-for-innovation/)
- Purves D, Augustine GJ, Fitzpatrick D, et , editors. Neuroscience. 2nd edition. Sunderland (MA): Sinauer Associates; 2001. The Enteric Nervous System. Available from: https://www.ncbi.nlm.nih.gov/books/NBK11097/
- Fleming MA 2nd, Ehsan L, Moore SR, Levin DE. The Enteric Nervous System and Its Emerging Role as a Therapeutic Gastroenterol Res Pract. 2020 Sep 8;2020:8024171. doi: 10.1155/2020/8024171. PMID: 32963521; PMCID: PMC7495222.
- Matthews, , ‘The second brain in your gut,’ Science Focus, September 8, 2020 https://www.sciencefocus.com/the-human-body/a-gut-feeling-meet-your-second- brain
- MacFabe Autism: Metabolism, mitochondria, and the microbiome. Glob Adv Health Med. 2013;2(6):52-66.
- Thursby E., Juge N. Introduction to the human gut microbiota. J. 2017;474:1823–1836. doi: 10.1042/BCJ20160510.
- O’Toole PW, Marchesi Regulation of innate immune function by microbiota. Microb Cell. 2017. doi:10.15698/mic2017.12.596
- Di Giacinto C et Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005. doi: 10.4049/jimmunol.174.6.3237
- Quevrain E et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016. doi: 10.1136/gutjnl-2015-310297
- Ganesh BP et al. Commensal akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic PLoS One. 2013. doi: 10.1371/journal.pone.0074963
- Lopetuso LR et Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 2013. doi: 10.1186/1757-4749-5-23
- Mazmanian SK et An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005. doi: 10.1016/j.cell.2005.07.007
- Arnold IC et Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology. 2011. doi: 10.1053/j.gastro.2011.06.006
- https://www.nccih.nih.gov/health/probiotics-what-you-need-to-know
- Chen Y, Xu J, Chen Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients. 2021 Jun 19;13(6):2099. doi: 10.3390/nu13062099. PMID: 34205336; PMCID: PMC8234057.
- Procaccini C, Pucino V, De Rosa V, Marone G and Matarese G (2014) Neuro-endocrine networks controlling immune system in health and Front. Immunol. 5:143. doi: 10.3389/fimmu.2014.00143
- Coates, , Lee, M.J., Norton, D., et. al. The Skin and Intestinal Microbiota and Their Specific Innate Immune Systems, Front. Immunol., 17 December 2019 Sec. Microbial Immunology Volume 10 – 2019 | https://doi.org/10.3389/fimmu.2019.02950
- Lambring CB, Siraj S, Patel K, Sankpal UT, Mathew S, Basha R. Impact of the Microbiome on the Immune System. Crit Rev Immunol. 2019;39(5):313-328. doi: 10.1615/CritRevImmunol.2019033233. PMID: 32422014; PMCID:
- De Luca F, Shoenfeld The microbiome in autoimmune diseases. Clin Exp Immunol. 2019 Jan;195(1):74-85. doi: 10.1111/cei.13158. PMID: 29920643; PMCID: PMC6300652.
- Mahajan P, Kulkarni A, Bandre A, Mahajan S, Subramanian S (2021) Role of Gut Microbiota in Autoimmune Diseases: A J Vaccines Immunol 6: 163. DOI: 10.29011/2575-789X.000163
- Ruffoli R, Giorgi FS, Pizzanelli C, et The chemical neuroanatomy of vagus nerve stimulation. J Chem Neuroanatomy. 2011;42:288–296.
- O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015 Jan 15;277:32-48. doi: 10.1016/j.bbr.2014.07.027. Epub 2014 Jul 29. PMID:25078296.
- Fung TC, Olson CA, Hsiao Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017 Feb;20(2):145-155. doi: 10.1038/nn.4476. Epub 2017 Jan 16. PMID: 28092661; PMCID: PMC6960010.
- Zhu X, Li B, Lou P, Dai T, Chen Y, Zhuge A, Yuan Y, Li L. The Relationship Between the Gut Microbiome and Neurodegenerative Diseases. Neurosci Bull. 2021 Oct;37(10):1510-1522. doi: 1007/s12264-021-00730-8. Epub 2021 Jul 3. PMID: 34216356; PMCID: PMC8490573.
- Reem Abdel-Haq, Johannes M. Schlachetzki, Christopher K. Glass, Sarkis K. Mazmanian; Microbiome–microglia connections via the gut–brain axis. J Exp Med 7 January 2019; 216 (1): 41–59. doi: https://doi.org/10.1084/jem.20180794
- Jeon S, Kim H, Kim J, Seol D, Jo J, Choi Y, Cho S, Kim H. Positive Effect of Lactobacillus acidophilus EG004 on Cognitive Ability of Healthy Mice by Fecal Microbiome Analysis Using Full-Length 16S-23S rRNA Metagenome Sequencing. Microbiol Spectr. 2022 Feb 23;10(1):e0181521. doi: 10.1128/spectrum.01815-21. Epub 2022 Jan 12. PMID: 35019699; PMCID:
- Miao, , Zhang, L., Wu, L. et al. Quadruple perovskite ruthenate as a highly efficient catalyst for acidic water oxidation. Nat Commun 10, 3809 (2019). https://doi.org/10.1038/s41467-019-11789-3
- Wang X., Wang B.-R., Zhang X.-J., Xu Z., Ding Y.-Q., and Ju G.. 2002. Evidences for vagus nerve in maintenance of immune balance and transmission of immune information from gut to brain in STM-infected rats. World J. Gastroenterol. 8:540–545. 10.3748/wjg.v8.i3.540
- Mitra, S., Dash, R., Nishan, A. A., Habiba, S. U., & Moon, I. S. (2023). Brain modulation by the gut microbiota: From disease to Journal of Advanced Research, 53, 153-173. https://doi.org/10.1016/j.jare.2022.12.001
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013 Sep;54(9):2325-40. doi: 10.1194/jlr.R036012. Epub 2013 Jul PMID: 23821742; PMCID: PMC3735932.
- Kunst C, Schmid S, Michalski M, Tümen D, Buttenschön J, Müller M, Gülow The Influence of Gut Microbiota on Oxidative Stress and the Immune System. Biomedicines. 2023 May 8;11(5):1388. doi: 10.3390/biomedicines11051388. PMID: 37239059; PMCID: PMC10216031.
- Zhao T, Wang H, Liu Z, Liu Y, DeJi, Li B, Huang X. Recent Perspective of Lactobacillus in Reducing Oxidative Stress to Prevent Disease. Antioxidants (Basel). 2023 Mar 21;12(3):769. doi: 3390/antiox12030769. PMID: 36979017; PMCID: PMC10044891.
- Abd Mutalib N, Syed Mohamad SA, Jusril NA, Hasbullah NI, Mohd Amin MCI, Ismail NH. Lactic Acid Bacteria (LAB) and Neuroprotection, What Is New? An Up-To-Date Systematic Review. Pharmaceuticals (Basel). 2023 May 7;16(5):712. doi: 10.3390/ph16050712. PMID: 37242494; PMCID: PMC10221206.
- Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and Therap Adv Gastroenterol. 2013 Jan;6(1):39-51. doi: 10.1177/1756283X12459294. PMID: 23320049; PMCID: PMC3539293.
- https://www.jax.org/news-and-insights/minute-to-understanding/what-are-telomer es#:~:text=Telomeres%20are%20structures%20made%20from,are%20required%20for%20cell%20division.
- Shammas Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care. 2011 Jan;14(1):28-34. doi: 10.1097/MCO.0b013e32834121b1. PMID: 21102320; PMCID: PMC3370421.
- Velando A, Noguera JC, Aira M, Domínguez Gut microbiome and telomere length in gull hatchlings. Biol Lett. 2021 Oct;17(10):20210398. doi: 10.1098/rsbl.2021.0398. Epub 2021 Oct 13. PMID: 34637637; PMCID: PMC8510700.
- El Maï M, Bird M, Allouche A, Targen S, Şerifoğlu N, Lopes-Bastos B, Guigonis JM, Kang D, Pourcher T, Yue JX, Ferreira MG. Gut-specific telomerase expression counteracts systemic aging in telomerase-deficient zebrafish. Nat Aging. 2023 May;3(5):567-584. doi: 10.1038/s43587-023-00401-5. Epub 2023 May 4. PMID: 37142828; PMCID: PMC10191862.
- Balan E, Decottignies A, Deldicque Physical Activity and Nutrition: Two Promising Strategies for Telomere Maintenance? Nutrients. 2018 Dec 7;10(12):1942. doi: 10.3390/nu10121942. PMID: 30544511; PMCID: PMC6316700.
- Bosco N, Noti M. The aging gut microbiome and its impact on host immunity. Genes 2021 Oct;22(5-6):289-303. doi: 10.1038/s41435-021-00126-8. Epub 2021 Apr 19. PMID: 33875817; PMCID: PMC8054695.
- Konturek PC, Harsch IA, Konturek K, Schink M, Konturek T, Neurath MF, Zopf Gut⁻Liver Axis: How Do Gut Bacteria Influence the Liver? Med Sci (Basel). 2018 Sep 17;6(3):79. doi: 10.3390/medsci6030079. PMID: 30227645; PMCID: PMC6165386.
- DeGruttola AK, Low D, Mizoguchi A, Mizoguchi E. Current Understanding of Dysbiosis in Disease in Human and Animal Inflamm Bowel Dis. 2016 May;22(5):1137-50. doi: 10.1097/MIB.0000000000000750. PMID: 27070911; PMCID: PMC4838534.
- Virzì GM, Mattiotti M, de Cal M, Ronco C, Zanella M, De Rosa S. Endotoxin in Sepsis: Methods for LPS Detection and the Use of Omics Techniques. Diagnostics (Basel). 2022 Dec 27;13(1):79. doi: 3390/diagnostics13010079. PMID: 36611371; PMCID: PMC9818564.
- https://my.clevelandclinic.org/health/diseases/24065-hyperammonemia
- Velasquez MT, Ramezani A, Manal A, Raj Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins (Basel). 2016 Nov 8;8(11):326. doi: 10.3390/toxins8110326. PMID: 27834801; PMCID: PMC5127123.
- Kenneth O’Riordan, Michael K. Collins, Gerard M. Moloney, et al., “Short-chain fatty acids: Microbial metabolites for gut-brain axis signaling,” Molecular and Cellular Endocrinology, Volume 546, 2022, 111572, ISSN 0303-7207, https://doi.org/10.1016/j.mce.2022.111572.
- Yang R, Qian L. Research on Gut Microbiota-Derived Secondary Bile Acids in Cancer Integr Cancer Ther. 2022 Jan-Dec;21:15347354221114100. doi: 10.1177/15347354221114100. PMID: 35880833; PMCID: PMC9421216.
- Baker JM, Al-Nakkash L, Herbst-Kralovetz Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas. 2017 Sep;103:45-53. doi: 10.1016/j.maturitas.2017.06.025. Epub 2017 Jun 23. PMID: 28778332.
- Foster JA, Rinaman L, Cryan Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. 2017 Mar 19;7:124-136. doi: 10.1016/j.ynstr.2017.03.001. PMID: 29276734; PMCID: PMC5736941.
- Madison A, Kiecolt-Glaser JK. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and Curr Opin Behav Sci. 2019 Aug;28:105-110. doi: 10.1016/j.cobeha.2019.01.011. Epub 2019 Mar 25. PMID: 32395568; PMCID: PMC7213601.
- Lee CJ, Sears CL, Maruthur N. Gut microbiome and its role in obesity and insulin resistance. Ann N Y Acad 2020 Feb;1461(1):37-52. doi: 10.1111/nyas.14107. Epub 2019 May 14. PMID: 31087391.
- https://www.cedars-sinai.org/newsroom/gut-bacteria-may-play-a-role-in-diabetes/ #:~:text=The%20study%2C%20published%20in%20the,to%20have%20lower%2 0insulin%20sensitivity.
- Han, H., Yi, B., Zhong, R. et al. From gut microbiota to host appetite: gut microbiota-derived metabolites as key Microbiome 9, 162 (2021). https://doi.org/10.1186/s40168-021-01093-y
- https://www.rupahealth.com/post/gut-brain-axis#:~:text=Gut%20Bacteria%20and%20Neurotransmitters%20Production,role%20in%20mood%20and%20cognition.
- https://www.ucsf.edu/news/2021/06/420891/caloric-restriction-alters-microbiome- enhancing-weight-loss-increasing#:~:text=Researchers%20at%20UC%20San%2 0Francisco,to%20severe%20diarrhea%20and%20colitis.
- Kesavelu D, Jog Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther Adv Infect Dis. 2023 Feb 24;10:20499361231154443. doi: 10.1177/20499361231154443. PMID: 36860273; PMCID: PMC9969474.
